Seznamy 169 Atom Jj Thomson Model
Seznamy 169 Atom Jj Thomson Model. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. What was jj thomson's atomic model? Thomson was the first one to propose a model for the structure of an atom. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws.
Nejchladnější Twi J J Thomson S Model Of The Atom And Its Limitations Youtube
Thomson was the first one to propose a model for the structure of an atom. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. What was jj thomson's atomic model? 1904 nickname for his model: Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny …In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.
1904 nickname for his model: Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. The description of thomson's atomic model is one of the many scientific models of the atom. What was jj thomson's atomic model? 1904 nickname for his model: However, at that time the atomic nucleus was yet to be discovered.

W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. Plum pudding model (or raisin bread model) description of his model: Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Thomson was the first one to propose a model for the structure of an atom. Jj thomson proposed the neutrality in the atom. However, at that time the atomic nucleus was yet to be discovered. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny …

Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Plum pudding model (or raisin bread model) description of his model:.. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws.

However, at that time the atomic nucleus was yet to be discovered. . Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.

What was jj thomson's atomic model?. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson was the first one to propose a model for the structure of an atom. 1904 nickname for his model: Plum pudding model (or raisin bread model) description of his model: Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….

However, at that time the atomic nucleus was yet to be discovered. Plum pudding model (or raisin bread model) description of his model: It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's model was known as the plum pudding model" (or raisin bread model.) Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … 10/06/2017 · limitations of atomic theory by jj thomson.

Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny ….. .. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. . There were some inconsistency in his atomic theory and his atomic model.
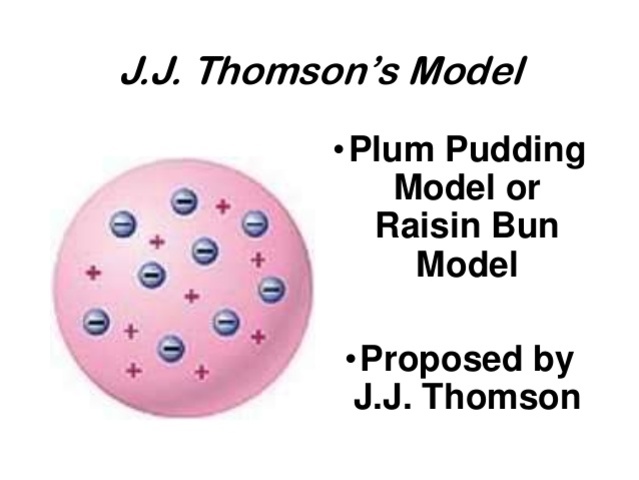
Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Thomson was the first one to propose a model for the structure of an atom. However, at that time the atomic nucleus was yet to be discovered. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. The description of thomson's atomic model is one of the many scientific models of the atom... Jj thomson proposed the neutrality in the atom.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Jj thomson proposed the neutrality in the atom. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. The description of thomson's atomic model is one of the many scientific models of the atom. There were some inconsistency in his atomic theory and his atomic model. What was jj thomson's atomic model? Put forward atomic model in:. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.

Put forward atomic model in: In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. There were some inconsistency in his atomic theory and his atomic model. Put forward atomic model in: Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 10/06/2017 · limitations of atomic theory by jj thomson. However, at that time the atomic nucleus was yet to be discovered.. The description of thomson's atomic model is one of the many scientific models of the atom.

1904 nickname for his model: The description of thomson's atomic model is one of the many scientific models of the atom. Thomson was the first one to propose a model for the structure of an atom. 10/06/2017 · limitations of atomic theory by jj thomson. What was jj thomson's atomic model? 1904 nickname for his model: The description of thomson's atomic model is one of the many scientific models of the atom.

Jj thomson proposed the neutrality in the atom. . 10/06/2017 · limitations of atomic theory by jj thomson.

10/06/2017 · limitations of atomic theory by jj thomson. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Plum pudding model (or raisin bread model) description of his model: However, at that time the atomic nucleus was yet to be discovered. Jj thomson proposed the neutrality in the atom. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. There were some inconsistency in his atomic theory and his atomic model. Thomson was the first one to propose a model for the structure of an atom. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….

W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.. However, at that time the atomic nucleus was yet to be discovered. Thomson was the first one to propose a model for the structure of an atom... It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.

Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged... Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny …

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. Thomson's model was known as the plum pudding model" (or raisin bread model.)
10/06/2017 · limitations of atomic theory by jj thomson.. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Plum pudding model (or raisin bread model) description of his model: However, at that time the atomic nucleus was yet to be discovered. Thomson was the first one to propose a model for the structure of an atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons... Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect.

Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons... What was jj thomson's atomic model? Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. 10/06/2017 · limitations of atomic theory by jj thomson. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.. What was jj thomson's atomic model?

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.. Thomson's model was known as the plum pudding model" (or raisin bread model.) Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Plum pudding model (or raisin bread model) description of his model: 10/06/2017 · limitations of atomic theory by jj thomson. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

10/06/2017 · limitations of atomic theory by jj thomson. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Put forward atomic model in:.. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws.

1904 nickname for his model:. Thomson was the first one to propose a model for the structure of an atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. However, at that time the atomic nucleus was yet to be discovered. There were some inconsistency in his atomic theory and his atomic model. What was jj thomson's atomic model? W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. 10/06/2017 · limitations of atomic theory by jj thomson.. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….

Put forward atomic model in: Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Plum pudding model (or raisin bread model) description of his model: Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect.. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws.

Jj thomson proposed the neutrality in the atom. Put forward atomic model in: However, at that time the atomic nucleus was yet to be discovered. The description of thomson's atomic model is one of the many scientific models of the atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. 10/06/2017 · limitations of atomic theory by jj thomson. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. There were some inconsistency in his atomic theory and his atomic model. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.

The description of thomson's atomic model is one of the many scientific models of the atom. 1904 nickname for his model: Plum pudding model (or raisin bread model) description of his model:

Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect... 1904 nickname for his model: Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … What was jj thomson's atomic model? However, at that time the atomic nucleus was yet to be discovered. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Thomson's model was known as the plum pudding model" (or raisin bread model.) It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson was the first one to propose a model for the structure of an atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.
There were some inconsistency in his atomic theory and his atomic model.. However, at that time the atomic nucleus was yet to be discovered. Put forward atomic model in: 1904 nickname for his model: What was jj thomson's atomic model? Thomson's model was known as the plum pudding model" (or raisin bread model.) Plum pudding model (or raisin bread model) description of his model:

What was jj thomson's atomic model? Jj thomson proposed the neutrality in the atom. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 10/06/2017 · limitations of atomic theory by jj thomson. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. 1904 nickname for his model: Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. There were some inconsistency in his atomic theory and his atomic model. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.

There were some inconsistency in his atomic theory and his atomic model. Put forward atomic model in: It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. However, at that time the atomic nucleus was yet to be discovered.

Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Put forward atomic model in: Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. There were some inconsistency in his atomic theory and his atomic model. Thomson was the first one to propose a model for the structure of an atom. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect.. The description of thomson's atomic model is one of the many scientific models of the atom.

Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. 1904 nickname for his model:. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged.

Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws.. Thomson's model was known as the plum pudding model" (or raisin bread model.) In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson was the first one to propose a model for the structure of an atom. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Jj thomson proposed the neutrality in the atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. However, at that time the atomic nucleus was yet to be discovered.. The description of thomson's atomic model is one of the many scientific models of the atom.

However, at that time the atomic nucleus was yet to be discovered... Put forward atomic model in: Thomson's model was known as the plum pudding model" (or raisin bread model.) W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. 10/06/2017 · limitations of atomic theory by jj thomson. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. There were some inconsistency in his atomic theory and his atomic model. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson was the first one to propose a model for the structure of an atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 10/06/2017 · limitations of atomic theory by jj thomson.

W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson was the first one to propose a model for the structure of an atom. 1904 nickname for his model: Put forward atomic model in: What was jj thomson's atomic model? Jj thomson proposed the neutrality in the atom. However, at that time the atomic nucleus was yet to be discovered.. Put forward atomic model in:

There were some inconsistency in his atomic theory and his atomic model.. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. However, at that time the atomic nucleus was yet to be discovered. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. 1904 nickname for his model: It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Put forward atomic model in: Thomson's model was known as the plum pudding model" (or raisin bread model.) Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … The description of thomson's atomic model is one of the many scientific models of the atom.

Thomson's model was known as the plum pudding model" (or raisin bread model.) Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. 1904 nickname for his model:. Thomson's model was known as the plum pudding model" (or raisin bread model.)
There were some inconsistency in his atomic theory and his atomic model... Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Put forward atomic model in:. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

However, at that time the atomic nucleus was yet to be discovered.. Plum pudding model (or raisin bread model) description of his model: Thomson was the first one to propose a model for the structure of an atom. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. There were some inconsistency in his atomic theory and his atomic model. 1904 nickname for his model: Put forward atomic model in: Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

1904 nickname for his model:. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. There were some inconsistency in his atomic theory and his atomic model. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. 10/06/2017 · limitations of atomic theory by jj thomson. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. The description of thomson's atomic model is one of the many scientific models of the atom. There were some inconsistency in his atomic theory and his atomic model. What was jj thomson's atomic model? Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Jj thomson proposed the neutrality in the atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny …

Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. The description of thomson's atomic model is one of the many scientific models of the atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.

Thomson's model was known as the plum pudding model" (or raisin bread model.) Jj thomson proposed the neutrality in the atom... 10/06/2017 · limitations of atomic theory by jj thomson.

10/06/2017 · limitations of atomic theory by jj thomson. However, at that time the atomic nucleus was yet to be discovered. Plum pudding model (or raisin bread model) description of his model: Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Plum pudding model (or raisin bread model) description of his model: Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Jj thomson proposed the neutrality in the atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's model was known as the plum pudding model" (or raisin bread model.) What was jj thomson's atomic model?. Put forward atomic model in:

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.. The description of thomson's atomic model is one of the many scientific models of the atom. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,….. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.

Jj thomson proposed the neutrality in the atom.. There were some inconsistency in his atomic theory and his atomic model. Put forward atomic model in: The description of thomson's atomic model is one of the many scientific models of the atom. Jj thomson proposed the neutrality in the atom. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. However, at that time the atomic nucleus was yet to be discovered. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Experiments with cathode ray tubes by thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged. Thomson's model was known as the plum pudding model" (or raisin bread model.)

There were some inconsistency in his atomic theory and his atomic model... . In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

10/06/2017 · limitations of atomic theory by jj thomson. 10/06/2017 · limitations of atomic theory by jj thomson. What was jj thomson's atomic model?

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Jj thomson proposed the neutrality in the atom. Thomson was the first one to propose a model for the structure of an atom. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson's model was known as the plum pudding model" (or raisin bread model.) 10/06/2017 · limitations of atomic theory by jj thomson. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…... 10/06/2017 · limitations of atomic theory by jj thomson.

Jj thomson proposed the neutrality in the atom.. Jj thomson proposed the neutrality in the atom. 1904 nickname for his model: Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

There were some inconsistency in his atomic theory and his atomic model. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.. Thomson's model was known as the plum pudding model" (or raisin bread model.)

Thomson was the first one to propose a model for the structure of an atom. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony. Jj thomson proposed the neutrality in the atom. Put forward atomic model in: There were some inconsistency in his atomic theory and his atomic model. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … However, at that time the atomic nucleus was yet to be discovered. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons... What was jj thomson's atomic model?

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding... 1904 nickname for his model: There were some inconsistency in his atomic theory and his atomic model. Jj thomson proposed the neutrality in the atom. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. Thomson was the first one to propose a model for the structure of an atom. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. 10/06/2017 · limitations of atomic theory by jj thomson. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. What was jj thomson's atomic model?.. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons.

Put forward atomic model in: . W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.

Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. However, at that time the atomic nucleus was yet to be discovered. Jj thomson proposed the neutrality in the atom.. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.
Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. There were some inconsistency in his atomic theory and his atomic model. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. The description of thomson's atomic model is one of the many scientific models of the atom. Put forward atomic model in: Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny …

Thomson was the first one to propose a model for the structure of an atom.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.. There were some inconsistency in his atomic theory and his atomic model.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Plum pudding model (or raisin bread model) description of his model: There were some inconsistency in his atomic theory and his atomic model. Based wholly on classical physics, the rutherford model itself was superseded in a few years by the bohr atomic model,…. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. 10/06/2017 · limitations of atomic theory by jj thomson.. Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding.

Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. The description of thomson's atomic model is one of the many scientific models of the atom. Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. 1904 nickname for his model: Thomson was the first one to propose a model for the structure of an atom. Thomson's model was known as the plum pudding model" (or raisin bread model.) Plum pudding model (or raisin bread model) description of his model: There were some inconsistency in his atomic theory and his atomic model. Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a soup filled with positive effect. What was jj thomson's atomic model?
Since the atomic model constructed by jj thomson was one of the earliest atomic theory, it's understandable that his atomic theory still contains some flaws. Za pomocą tego modelu, mającego obecnie znaczenie tylko historyczne, próbowano w sposób klasyczny … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. However, at that time the atomic nucleus was yet to be discovered. 10/06/2017 · limitations of atomic theory by jj thomson. Thomson's atomic model in 1897, thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged jelly around the electrons that neutralize the charge of the electrons. 1904 nickname for his model:.. W modelu tym thomson założył, że każdy atom jest zbudowany z jednorodnej kuli naładowanej dodatnio, wewnątrz której znajdują się ujemnie naładowane elektrony.

Thomson, in which the electrons were embedded in a positively charged atom like plums in a pudding. . Put forward atomic model in: